by theglamscientist | | chemistry |
As usual, I was browsing my twitter timeline when I came across something that made my eyebrows raise. What I read reminded me of how little “normal” people know about chemistry. By normal, I mean people that aren’t as nerdily passionate about chemistry as I. Although I’m a cosmetic chemist, I enjoy and have studied all areas of chemistry which leads me to this pet peeve…
The elements in a compound alone do not determine the properties of the compound.
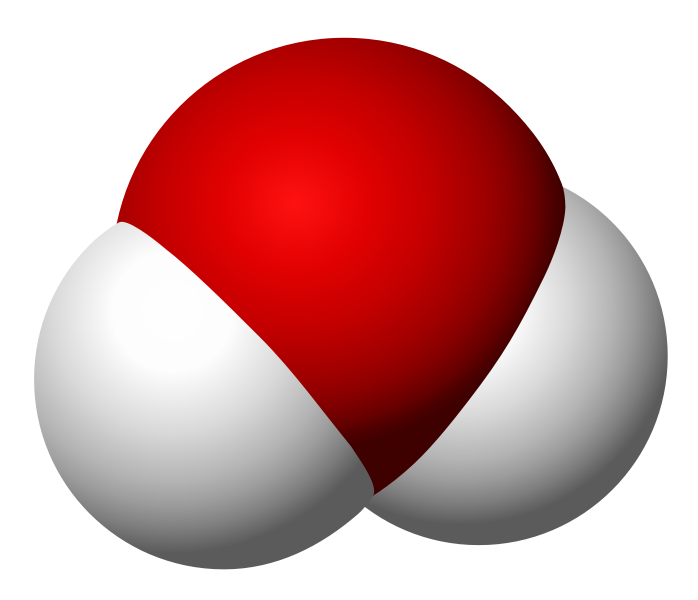
I want to keep this quick, so allow me to use an example. Take water and peroxide, for instance. Notice that they have unique names, yet they are made of the same 2 elements– Hydrogen and Oxygen. What is the difference between the two? Well, the number of molecules for starters. Water contains 2 molecules of hydrogen per 1 molecule of Oxygen hence the notation H2O. Peroxide, on the other hand, contains 2 molecules of Hydrogen per 2 molecules of Oxygen and is noted as H2O2. They also differ in structure. The molecules in water occupy the same plane, however, peroxide molecules occupy 2 planes. **To visualize the planes consider a sheet of paper. In a water molecule, everything lays flat on the sheet of paper. However, in a peroxide molecule half of the compound lays flat on the paper and the other half sticks out of the paper.
Water Molecule
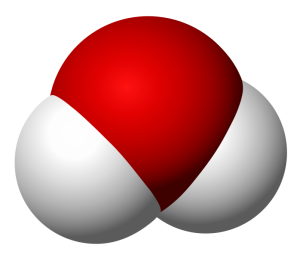
Peroxide Molecule
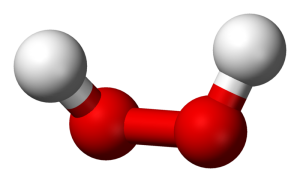
The number of molecules and their structure/arrangement in the compound make water and hydrogen peroxide very different. In fact, while water is safe to ingest and apply to skin, the same is not true for peroxide. In low concentrations, peroxide can be applied to the skin and even swished around in your mouth. BUT in higher concentrations, H2O2 is highly corrosive and will cause serious chemical burns to your skin and mouth. One job I held in undergrad required me to work with very concentrated peroxide. I had to be very careful to properly dilute it to prevent injury to myself and those using the finished product. H2O2 is NO JOKE!
I used the example of water and peroxide because it was among the simplest I could think of. However, these types of major differences can be seen between all kinds of compounds that happen to share elements or similar structures. Keep this in mind when assessing your cosmetics ingredients or when you see things like “Propylene Glycol is used in anti-freeze. It can’t possibly be safe in cosmetics.” Give me a break! A lot must be considered in the assessment of chemical safety including stuff like steric tension, hydrogen bonds, concentration… You get the picture.
That’s enough of my nerdy pet peeve… Here’s to the Glam Life!
by theglamscientist | | chemicals, chemistry, cosmetics, hair care, skin care |

This is the very first Um, What?! Wednesday! Every Wednesday I’m going to break down one ingredient commonly found in personal care products; giving you the good, the bad and the ugly. Today its all about EDTA, my absolute favorite chemical (don’t ask me why, I’m a nerd!). Here are the facts:
EDTA and its salts– anything listed that has EDTA somewhere in the name– is found in just about all skin care treatments including lotions, creams, body wash, soap, shampoos and conditioners. Its official chemical name is (insert deep breath here) ethylenediamine tetraacetic acid. Its defining quality is the ability to bind metal ions. Popular applications in your personal care products are preservative and foaming/lather agent. As a preservative it prevents deterioration of the product, protects the fragrance, and prevents rancidity (common in products whose ingredients include water). It combines with metals such as calcium, magnesium and iron to achieve foam or lather. EDTA also keeps metals from being deposited on your hair, skin and scalp (cleanliness is of utmost importance right?).
Not only is this chemical known for getting the job done, its also been deemed safe by the FDA for food and cosmetic applications. That’s right, its safe enough to eat!!!! In reference to cosmetic and personal care applications, it is not a skin irritant or sensitizer, nor is it a carcinogen (won’t cause cancer). It results in very little skin penetration, so if found in your blood stream, its only in trace amounts.
I absolutely love this chemical!!!! See you next Wednesday!
by theglamscientist | | chemicals, chemistry, cosmetics, eco, environment, green, science |
I stumbled upon an article in an issue of Cosmetiscope– a journal for cosmetic scientists– detailing what ‘green’ means in the cosmetic industry. What I once thought to be no more than an eco-fad has quickly become quite important to a great majority internationally. Here’s what I learned…
In order to be considered green the cosmetic manufacturer must make an effort to prevent waste. Generally chemical waste is detrimental to the environment so you can see why this would be important. A few other requirements are linked to preventing or reducing waste, so I’ll discuss them now. Any substance that is used for the sole purpose of making a reaction work has to be renewable. It cannot be ‘used up’ in the process and it cannot be inactive when the process is complete. Similarly, green processes minimize the creation of side products that cannot be used in other applications. In addition, green processes make use of what I would call personal assistants which speed up the reaction and minimize the amount of starting material needed.
Next on the chopping block– unnecessary chemicals. All the ingredients tossed into the mixing bowl have to be needed for effective application of the product. In other words, all those 15-35 letter ingredients that fascinate me so much (nerdy I know!) have to be in there for a darn good reason! Additionally, a fantastically green product serves its purpose and then calls it quits leaving no chemical scars on our blessed environment. And just for good measure, a real-time pollution prevention tool should be in place. Toxicity and risk of chemical disaster– like explosions, fires, gas leaks (no big deal, right?) are kept to a minimum.
And with all the talk about energy conservation locally and on capital hill, its no mystery why the cosmetic industry would strive to be particular about the energy use in their chemical processes.
Stay tuned to see how well your cosmetic company of choice is doing with the transition!




