As usual, I was browsing my twitter timeline when I came across something that made my eyebrows raise. What I read reminded me of how little “normal” people know about chemistry. By normal, I mean people that aren’t as nerdily passionate about chemistry as I. Although I’m a cosmetic chemist, I enjoy and have studied all areas of chemistry which leads me to this pet peeve…
The elements in a compound alone do not determine the properties of the compound.
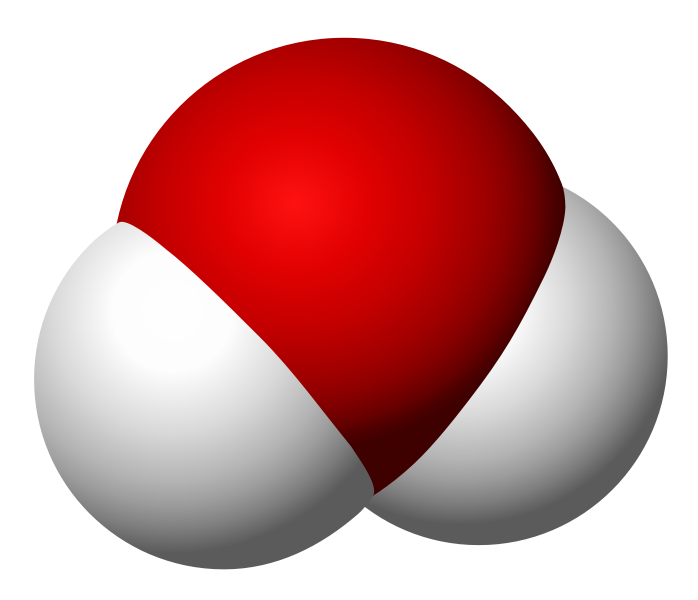
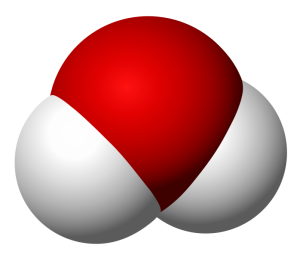
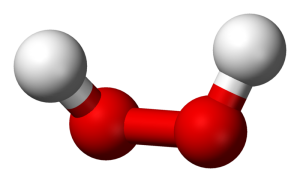
I want to keep this quick, so allow me to use an example. Take water and peroxide, for instance. Notice that they have unique names, yet they are made of the same 2 elements– Hydrogen and Oxygen. What is the difference between the two? Well, the number of molecules for starters. Water contains 2 molecules of hydrogen per 1 molecule of Oxygen hence the notation H2O. Peroxide, on the other hand, contains 2 molecules of Hydrogen per 2 molecules of Oxygen and is noted as H2O2. They also differ in structure. The molecules in water occupy the same plane, however, peroxide molecules occupy 2 planes. **To visualize the planes consider a sheet of paper. In a water molecule, everything lays flat on the sheet of paper. However, in a peroxide molecule half of the compound lays flat on the paper and the other half sticks out of the paper.
The number of molecules and their structure/arrangement in the compound make water and hydrogen peroxide very different. In fact, while water is safe to ingest and apply to skin, the same is not true for peroxide. In low concentrations, peroxide can be applied to the skin and even swished around in your mouth. BUT in higher concentrations, H2O2 is highly corrosive and will cause serious chemical burns to your skin and mouth. One job I held in undergrad required me to work with very concentrated peroxide. I had to be very careful to properly dilute it to prevent injury to myself and those using the finished product. H2O2 is NO JOKE!
I used the example of water and peroxide because it was among the simplest I could think of. However, these types of major differences can be seen between all kinds of compounds that happen to share elements or similar structures. Keep this in mind when assessing your cosmetics ingredients or when you see things like “Propylene Glycol is used in anti-freeze. It can’t possibly be safe in cosmetics.” Give me a break! A lot must be considered in the assessment of chemical safety including stuff like steric tension, hydrogen bonds, concentration… You get the picture.
That’s enough of my nerdy pet peeve… Here’s to the Glam Life!